A11 - Functional analysis of septin scaffolds assembled at the plasma membrane during cytokinesis
Principal Investigators
Prof. Dr. Michael Krauß, FMP Berlin
Prof. Dr. Helge Ewers, FU Berlin
Septin GTPases constitute membrane-associated scaffolds that orchestrate the recruitment of soluble factors and form diffusion barriers spatially confining membrane subdomains. Although septins have been strongly correlated with disease, such as cancer, surprisingly little is known about respective binding partners and molecular mechanisms underlying pathogenesis.
We have recently identified SEPT9 as a binding partner of CIN85, an adaptor protein regulating endosomal trafficking of various growth factor receptors. Work in the past funding period revealed that cancer-related isoforms of SEPT9 control surface levels of epidermal growth factor receptor (EGFR). We unraveled the underlying molecular mechanism by demonstrating that SEPT9-containing filaments are recruited to ligand-engaged EGFR at the plasma membrane (PM) in a CIN85-dependent manner. We also showed that SEPT9 competes with binding of the ubiquitin ligase Cbl, inhibits ubiquitination of EGFR, and negatively controls degradative sorting.
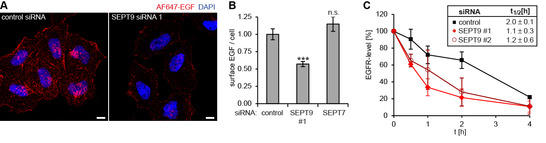
Figure 1: SEPT9 controls endosomal trafficking of EGFR (A/B) Surface-bound Alexa Fluor (AF)647-EGF. (B) Quantification of images as depicted in (A) by epifluorescence microscopy. (C) Degradation of EGFR in control or SEPT9 knockdown cells.
In a second project, we found select septin isoforms to interact with a particular PI(4,5)P-synthesizing enzyme. Our data demonstrate that this PI kinase is essential for stabilizing septin filaments at the plasma membrane and implicate this kinase in the coordination of store-operated Ca2+ entry, a process crucially dependent on the formation of junctions between the ER and the PM. We thus hypothesize that septin scaffolds spatiotemporally organize the synthesis of PI(4,5)P2 at ER-PM contact sites.
Work in the next funding period will focus on the functional interplay between septins and PI metabolism during the formation of intermembrane junctions. We will use live cell imaging approaches in combination with TIRF microscopy to study the contribution of PI(4,5)P2 synthesis at ER-PM contact sites stabilized by septin scaffolds. Furthermore, we will use our experience in determining structures of septin-related proteins to gain structural insight into complexes of the septin G domain with peptides/domains derived from the PI(4,5)P2-synthesizing kinase. Structure-derived mutants will be tested in rescue experiments to deduce the function of these interactions on the formation of membrane contact sites. Moreover, ultrastructural analyses will be applied to explore the spatial organization of the proteins under study. Finally, we will investigate the impact of functional contact sites on cellular signaling and ER function. Results of this project will elucidate the mechanisms underlying the formation and function of septin scaffolds and the molecular determinants of septin-related diseases.
References:
- Zehtabian A, Müller PM, Goisser M, Obendorf L, Jänisch L, Hümpfer N, Rentsch J, Ewers H. Precise measurement of nanoscopic septin ring structures with deep learning-assisted quantitative superresolution microscopy. Mol Biol Cell. 33(8) (2022)
- Geertsema HJ, Aimola G, Fabricius V, Fuerste JP, Kaufer BB, Ewers H. Left-handed DNA-PAINT for improved super-resolution imaging in the nucleus. Nat Biotechnol. 39(5):551-554 (2021)
- Gao M, Thielhorn R, Rentsch J, Honigmann A, Ewers H. Expansion STED microscopy (ExSTED). Methods Cell Biol. 161:15-31 (2021)
- Li JH, Santos-Otte P, Au B, Rentsch J, Block S, Ewers H. Directed manipulation of membrane proteins by fluorescent magnetic nanoparticles. Nat Commun.11(1):4259 (2020)
- Banko M, Mucha-Kruczynska I, Weise C, Heyd F, Ewers H. A homozygous genome-edited Sept2-EGFP fibroblast cell line. Cytoskeleton (Hoboken) 76(1):73-82 (2019)
-
Reubold, T.F., Faelber, K., Plattner, N., Posor, Y., Ketel, K., Curth, U., Schlegel, J., Anand, R., Manstein, D.J., Noé, F., Haucke, V., Daumke , O. & Eschenburg, S. Crystal structure of the dynamin tetramer. Nature 525, 404-408 (2015)
-
Diesenberg, K., Beerbaum, M., Fink,Krauss, M. Septin 9 negatively regulates ubiquitin-dependent downregulation of epidermal growth factor receptor. J Cell Sci 128, 397-407 (2015)
-
Wilhelm, B.G., Mandad, S., Truckenbrodt, S., Krohnert, K., Schafer, C., Rammner, B., Koo, S.J., Classen, G.A., Krauss, M., Haucke, V., Urlaub, H. & Rizzoli, S.O. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344, 1023-1028 (2014)
-
Schwefel, D., Arasu, B.S., Marino, S.F., Lamprecht, B., Kochert, K., Rosenbaum, E., Eichhorst, J., Wiesner, B., Behlke, J., Rocks, O., Mathas, S. & Daumke, O. Structural insights into the mechanism of GTPase activation in the GIMAP family. Structure 21, 550-559 (2013)